Go choke on a stack of pancakes. I don't usually agree with Irishlass but they have a right to free speech. Just like you...New Community Guideline: If you're a Nazi cunt you should kill yourself and anyone who doesn't tell you to kill yourself is being a useless pussy
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Musk's Illegal Activities
- Thread starter HipKat
- Start date
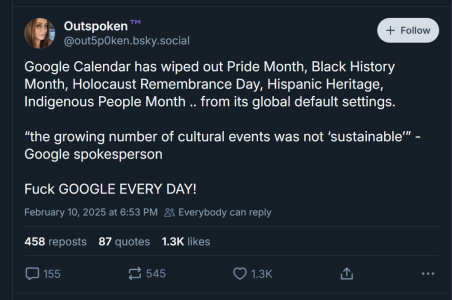
Outspoken™️ (@out5p0ken.bsky.social)
Google Calendar has wiped out Pride Month, Black History Month, Holocaust Remembrance Day, Hispanic Heritage, Indigenous People Month .. from its global default settings. “the growing number of cultural events was not ‘sustainable’” - Google spokesperson Fuck GOOGLE EVERY DAY!
The fact that Elon is behind the Resolute Desk on Time Magazine doesn't seem so "fake" now does it?
njbuff
Well-known member
Go choke on a stack of pancakes. I don't usually agree with Irishlass but they have a right to free speech. Just like you...
A stack of pancakes don’t stand a chance against a hate-filled whale.
njbuff
Well-known member
You’re a fuckin moron. Period. End of story.Trump brings hate, stupidity and lawlessness. njbuff follows in lockstep.
This week in TrumplandiaYou’re a fuckin moron. Period. End of story.
- Signed an Executive order that only he and his AG can determine what the law is
- Claimed that Free Speech was never part of the US Constitution.
- His House GOP passed a bill to cut 1 TRILLION dollars from Medicaid and Food Stamps (SNAP)
- Fired 300 Nuclear Regulators who oversee the nation's Nuclear Power
- Claimed that Ukraine started the war with Russia
DOGE Purges FDA Workers Reviewing Elon Musk’s Neuralink Brain Chip

DOGE Purges FDA Workers Reviewing Elon Musk’s Neuralink Brain Chip
The department has worked at breakneck speed to gut federal programs and cut tens of thousands of government workers.
The department has worked at breakneck speed to gut federal programs and cut tens of thousands of government workers.
Federal scientists reviewing Elon Musk’s Neuralink brain chip were fired as part of the Department of Government Efficiency’s latest purge, a report revealed Monday.The group of 20, from the Food and Drug Administration’s office of neurological and physical medicine devices, were abruptly axed over the weekend as “part of a broader purge of the federal workforce,” sources told Reuters.
DOGE and its young staffers have moved at breakneck speed to cut federal spending at the expense of tens of thousands of jobs in the federal government.
Critics have claimed Musk’s team should not have the power to make such sweeping firings in part because his businesses, including SpaceX and Tesla, have a number of contracts with the federal government.
This weekend’s FDA firings again sparked conflict of interest discussions with Musk at the helm of DOGE. Reuters reported alarm bells are already going off within the medical community.
“It’s intimidating to the FDA professionals who are overseeing Neuralink’s trial,” said Victor Krauthamer, a former FDA official for three decades, told the wire service. “We should be worried about the whole trial, and the protection of the people in the trial.”
President Donald Trump has said Musk would excuse himself from any conflicts of interest at DOGE. It is unclear if Musk had a personal say in the FDA scientists’ firing, but two sources in the agency told Reuters they do not believe their work on Neuralink specifically led to them being targeted.
The latest cuts come amid a purge of about 5,200 workers in the U.S. Department of Health and Human Services. The cuts largely impacted probationary employees—workers who have been in their position for less than two years who do not have the same protections as senior workers.
Neuralink is actively undergoing FDA trials. It promotes itself as allowing people with paralysis and other physical disabilities to control things in their environment using only their thoughts, but has faced headwinds in ensuring its users safety.
Blondie
Well-known member
This is outlandishly false.
Ok, yes, but I just don’t want to listen to people blaming the Democrats, when we have to do everything to help support them. The constant criticism of the left without the equal criticism of the right is half this country’s problem. The democrats have never done anything CLOSE to what Trump and the MAGA party IS ACTIVELY doing.This is outlandishly false.
ICRockets
Well-known member
What the fuck is there to support? They're not just NOT DOING ANYTHING, they're COMPLAINING THAT PEOPLE WANT THEM TO.Ok, yes, but I just don’t want to listen to people blaming the Democrats, when we have to do everything to help support them. The constant criticism of the left without the equal criticism of the right is half this country’s problem. The democrats have never done anything CLOSE to what Trump and the MAGA party IS ACTIVELY doing.
I wish I could dispute this, but I cannot unless they're being secretive about mounting some kind've defense against what is happening. The first problem is that when the Republicans won and started accusing the Democrats of being out of touch, etc, instead of answering back, the weak party leaders agreed! Eyerollingly stupid and a sign of just how weak they areWhat the fuck is there to support? They're not just NOT DOING ANYTHING, they're COMPLAINING THAT PEOPLE WANT THEM TO.
Blondie
Well-known member
I get your frustration, I really do, you need to build your algorithm.. it would be most dope bitchWhat the fuck is there to support? They're not just NOT DOING ANYTHING, they're COMPLAINING THAT PEOPLE WANT THEM TO.
ICRockets
Well-known member
I have no idea what happened in the 2nd half of this comment lmaoI get your frustration, I really do, you need to build your algorithm.. it would be most dope bitch
I was thinking the same thing!I have no idea what happened in the 2nd half of this comment lmao
Blondie
Well-known member
I was listening to Mac Miller and had eaten edibles .. LOLI have no idea what happened in the 2nd half of this comment lmao
Blondie
Well-known member
Wait I remember .. so Elon bought Twitter so that he could have influence over ALL the subscribers .. a "digital army" .. that is what YOU need, is a digital army for building the Democrats into a more powerful party again .. my thought process last night was somewhere along those linesI have no idea what happened in the 2nd half of this comment lmao